Oligonucleotide API Market Comprehensive Analysis, Share, Challenges, Business Opportunities to 2030
Published by mark itwired
Posted on September 2, 2021
8 min readLast updated: January 21, 2026

Published by mark itwired
Posted on September 2, 2021
8 min readLast updated: January 21, 2026

According to FMI – an ESOMAR-certified market research firm, the global oligonucleotide API market is estimated to reach US$ 3 Bn in 2020, and witness a CAGR of 11% through 2030.
According to FMI’s analysis, as of now, 100+ oligonucleotide APIs are in the clinical trials’ phase, and regulatory approval is on the cards. Post-approval, the demand for production capacity will witness an exponentiation, thereby driving the oligonucleotide API market through 2030.
Oligonucleotides are being looked upon as the subsequent large group of therapeutics following biologics and molecules. This could be attributed to the assurance on their part to develop drugs at lower costs.
Apart from immune therapy, microbial and cardiovascular infections, and cancer, oligonucleotide APIs are being tried out to treat neurological disorders like Alzheimer’s as well. However, factors like regulatory complexities and timely delivery of oligonucleotides (amidst Covid-19) are acting as restraints.
Request Report Sample @ https://www.futuremarketinsights.com/reports/sample/rep-gb-11933
FMI has analyzed players such as Akcea Therapeutics, Biogen, Sarepta Therapeutics, Jazz Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., and Dynavax Technologies in this report. According to the analysis, these players are consolidating their positions through new product launches.
For instance –
“Inorganic mode of growth coupled with broad therapeutic applications in gene therapy is expected to bolster the oligonucleotide API market” says the FMI analyst.
Synthetic oligonucleotides are being used in gene therapy for inactivating genes that help in propagating the disease. Antisense oligonucleotide APIs are used for disrupting the faulty gene’s transcription.
Also, siRNA could be used for signaling the cell to disrupt faulty mRNA’s translation. Along these lines, Pharmamar, in Jan 2020, signed an agreement with Jazz Pharmaceuticals for selling the API for “lurbinectedin”, one of the late-phase treatments for SCLC (small cell lung cancer) so that the latter could commercialize it.
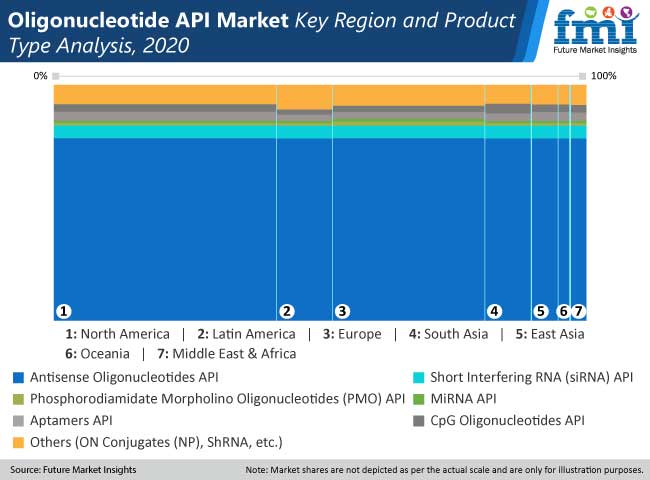
The study provides compelling insights on Oligonucleotide API market on basis of API type in detail cover every aspects of the market such as Antisense Oligonucleotides API, Short Interfering RNA (siRNA) API, Phosphorodiamidate Morpholino Oligonucleotides (PMO) API, MiRNA API, Aptamers API, CpG Oligonucleotides API and Others (ON Conjugates (NP), ShRNA, etc.), Marketing Status (Marketed, Clinical Trials (Clinical Phases)), and end users (Pharmaceutical Companies and Biotechnology Companies)) across seven major regions.
1.1. Global Market Outlook
1.2. Demand Side Trends
1.3. Supply Side Trends
1.4. Technology Assessment
1.5. Analysis and Recommendations
2.1. Market Coverage / Taxonomy
2.2. Market Definition / Scope / Limitations
2.3. Inclusions and Exclusions
3.1. Key Trends Impacting the Market
3.2. Product Innovation / Development Trends
4.1. Macro-Economic Factors
4.1.1. Global GDP Growth Outlook
4.1.2. Global Healthcare Outlook
4.2. Forecast Factors – Relevance & Impact
4.2.1. Research and Developmental Activities
4.2.2. Product Formulation Advancement
4.2.3. Key Players Historical Growth
4.2.4. Market Consolidation Activities
4.2.5. Growing Investment by Key Players in Product Development
4.3. Current COVID19 Statistics and Probable Future Impact
4.3.1. Current GDP Projection and Probable Impact
4.3.2. Current Economic Projection as compared to 2008 financial analysis
4.3.3. COVID19 and Impact Analysis
4.3.4. 2020 Market Scenario
4.3.5. Recovery Scenario – Short term, Midterm and Long Term Impact
4.4. Market Dynamics
4.4.1. Drivers
4.4.2. Restraints
4.4.3. Opportunities
5.1. Product USPs/ Features
5.2. Product Adoption / Usage Analysis
5.3. Regulatory Scenario
5.4. Opportunity Analysis For The Products Under Late Stage Clinical Trial
5.5. Revenue Breakdown by Region by Approved Market Products
5.6. Supply-Chain Analysis
5.7. Value Chain Analysis
The post Oligonucleotide API Market Comprehensive Analysis, Share, Challenges, Business Opportunities to 2030 first appeared on Market Research Blog.
According to FMI – an ESOMAR-certified market research firm, the global oligonucleotide API market is estimated to reach US$ 3 Bn in 2020, and witness a CAGR of 11% through 2030.
According to FMI’s analysis, as of now, 100+ oligonucleotide APIs are in the clinical trials’ phase, and regulatory approval is on the cards. Post-approval, the demand for production capacity will witness an exponentiation, thereby driving the oligonucleotide API market through 2030.
Oligonucleotides are being looked upon as the subsequent large group of therapeutics following biologics and molecules. This could be attributed to the assurance on their part to develop drugs at lower costs.
Apart from immune therapy, microbial and cardiovascular infections, and cancer, oligonucleotide APIs are being tried out to treat neurological disorders like Alzheimer’s as well. However, factors like regulatory complexities and timely delivery of oligonucleotides (amidst Covid-19) are acting as restraints.

Request Report Sample @ https://www.futuremarketinsights.com/reports/sample/rep-gb-11933
FMI has analyzed players such as Akcea Therapeutics, Biogen, Sarepta Therapeutics, Jazz Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., and Dynavax Technologies in this report. According to the analysis, these players are consolidating their positions through new product launches.
For instance –
“Inorganic mode of growth coupled with broad therapeutic applications in gene therapy is expected to bolster the oligonucleotide API market” says the FMI analyst.
Synthetic oligonucleotides are being used in gene therapy for inactivating genes that help in propagating the disease. Antisense oligonucleotide APIs are used for disrupting the faulty gene’s transcription.
Also, siRNA could be used for signaling the cell to disrupt faulty mRNA’s translation. Along these lines, Pharmamar, in Jan 2020, signed an agreement with Jazz Pharmaceuticals for selling the API for “lurbinectedin”, one of the late-phase treatments for SCLC (small cell lung cancer) so that the latter could commercialize it.
The study provides compelling insights on Oligonucleotide API market on basis of API type in detail cover every aspects of the market such as Antisense Oligonucleotides API, Short Interfering RNA (siRNA) API, Phosphorodiamidate Morpholino Oligonucleotides (PMO) API, MiRNA API, Aptamers API, CpG Oligonucleotides API and Others (ON Conjugates (NP), ShRNA, etc.), Marketing Status (Marketed, Clinical Trials (Clinical Phases)), and end users (Pharmaceutical Companies and Biotechnology Companies)) across seven major regions.
1.1. Global Market Outlook
1.2. Demand Side Trends
1.3. Supply Side Trends
1.4. Technology Assessment
1.5. Analysis and Recommendations
2.1. Market Coverage / Taxonomy
2.2. Market Definition / Scope / Limitations
2.3. Inclusions and Exclusions
3.1. Key Trends Impacting the Market
3.2. Product Innovation / Development Trends
4.1. Macro-Economic Factors
4.1.1. Global GDP Growth Outlook
4.1.2. Global Healthcare Outlook
4.2. Forecast Factors – Relevance & Impact
4.2.1. Research and Developmental Activities
4.2.2. Product Formulation Advancement
4.2.3. Key Players Historical Growth
4.2.4. Market Consolidation Activities
4.2.5. Growing Investment by Key Players in Product Development
4.3. Current COVID19 Statistics and Probable Future Impact
4.3.1. Current GDP Projection and Probable Impact
4.3.2. Current Economic Projection as compared to 2008 financial analysis
4.3.3. COVID19 and Impact Analysis
4.3.4. 2020 Market Scenario
4.3.5. Recovery Scenario – Short term, Midterm and Long Term Impact
4.4. Market Dynamics
4.4.1. Drivers
4.4.2. Restraints
4.4.3. Opportunities
5.1. Product USPs/ Features
5.2. Product Adoption / Usage Analysis
5.3. Regulatory Scenario
5.4. Opportunity Analysis For The Products Under Late Stage Clinical Trial
5.5. Revenue Breakdown by Region by Approved Market Products
5.6. Supply-Chain Analysis
5.7. Value Chain Analysis
The post Oligonucleotide API Market Comprehensive Analysis, Share, Challenges, Business Opportunities to 2030 first appeared on Market Research Blog.
Explore more articles in the Research Reports category











